Anisotropy :
It is the ability of crystalline solids to change their physrcal properties when measured In drfferen
directions. The property is due to different arrangment of constituents in different directions. Different types 0f
particles fall on the way of measurements in different directions. Hence composition of crystalline solid
changes with directions, changing physical propertie
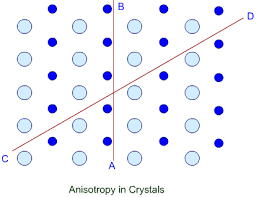
Different arrangement of constituent particles about different directions AB, CD and EF.
Isotropy : The ability amorphous solids to exhibit identical physical properties even through measured in different direction .
in amorphous solid order of regular pattern of arrangement is to short range. Hence arrangement is irregular along all directions. Due to which magnitude of physical property is identical along all directions.
Isomorphous :
When two or more crystalline substances have the same crystalline structure, they are said to be isomorphous and phenomenon is isomorphism. '
e.g. NaCl & KCl; KZSO‘ & KZSeO‘, NaN03 & CaCOJ, NaF & MgO, Cr203 & Fe 0
Polymorphous:
a single substance whitch crystalline in two or more forms under different cinditions of solidification is called polymorphous and phenomenon is polymorphism. These polymorphic forms are also called allotropic forms or allotropes. ‘
e.g. Carbon exists as diamond and graphite
Sulphur exists as rhombic and monoclinic.
Glass is a homogeneous mixture ofSiOz, NaIO, boron oxide (B203) and transition metaloxide to impart colour.
It is optically transparent material.
About 800 ditlerent types of glasses are manufactured by changing the composition. Quartz glass is obtained only from SIO2
Pyrex glass is obtained by fusing together 60-80% SiO2, 10-25% B2O3 and remaining amount of AL2O3. Glass is amorphous solid is considered as a super cooled liquid.
Sodalime glass : 75% SiOz, 15% N320 and 10% C30
Red glass : SiO2 and trace amount of gold & copper.
Yellow glass : SiO2 + U02
Blue glass : SiOz + CaO or CuO
Green glass : SiO2 + FeZO3 or CuO
No comments:
Post a Comment